By: Sridevi R. Pitta, MD, MBA, FSCAI and Rahul Sharma, MD, FSCAI
Transradial access (TRA) for both diagnostic and interventional cardiovascular procedures is growing rapidly worldwide and in the U.S. Newer data suggest that the transradial approach is superior to the transfemoral approach in reducing all-cause mortality, vascular complications, and major bleeding events when coronary angiography or percutaneous coronary intervention (PCI) is performed in patients with acute coronary syndrome (ACS).1
Radial artery occlusion (RAO) is the most common vascular complication of TRA but is rarely associated with major clinical consequences such as hand ischemia, given the dual blood supply to the hand. Following TRA best practices and strategies to minimize the incidence of RAO allows for future use of the radial artery for cardiac catheterization and potentially for use in coronary artery bypass surgery and arteriovenous (AV) fistula creation for dialysis access. In this SCAI Quality Improvement Committee “Tip of the Month,” we present pharmacologic and nonpharmacologic strategies for prevention of RAO as well as management options after RAO.
In a meta-analysis of 66 studies, the incidence of RAO was found to be 11 percent with a 6F sheath vs. 2 percent with the use of a 5F sheath. RAO decreased from 7.7 percent within 24 hours post-procedure to 5.5 percent after one week, due to the recanalization of the artery from the activation of primary fibrinolysis. The same study showed that the incidence of RAO is lower in the setting of PCI compared to diagnostic angiography (4.5 percent vs. 8.8 percent, p<0.001) due to the routine use of antiplatelet and anticoagulant therapy with PCI.2 Baseline patient characteristics, procedural variables, and the post-procedure hemostasis protocol can influence the incidence of RAO.
Table 1

RAO Prevention
Pharmacologic Strategies:
- Intraprocedural administration of anticoagulant agents: Both intra-arterial and intravenous unfractionated heparin (UFH) are equally effective in preventing RAO, based on randomized data.3 A protective effect against RAO was also noted when bivalirudin was administered.4 Published data supports a dose-dependent effect of UFH in the prevention of RAO, with 5,000 units being the most effective dose, while in obese patients, a weight-based approach may be valid with a dosing strategy of 50 units/kg.
- Prevention of a radial artery spasm: The standard practice of preventing a radial artery spasm, with use of intra-arterial nitroglycerin or nondihydropyridine calcium channel blockers such as verapamil, lowers the incidence of RAO. Verapamil in combination with nitroglycerin or 5 mg of verapamil alone are the most effective measures to reduce a spasm. The use of post-procedural/pre-hemostasis intra-arterial nitroglycerin reduced the incidence of RAO compared to a placebo (8.3 percent vs. 11.7 percent, p=0.006).5
Nonpharmacologic Strategies:
- Minimize the sheath-to-radial artery ratio (SRAR): A larger SRAR predisposes to RAO. Use 4F or 5F catheters for diagnostic angiography in patients with small caliber radial arteries.6 Although not formally studied as a strategy to reduce RAO, pre-procedure evaluation of the right and left radial arteries with a bedside ultrasound can be considered to choose the radial artery with the larger diameter. The use of newer 6F sheaths with smaller outer diameters and sheathless guiding catheters are other ways to lower the SRAR and potentially the risk of RAO.
- Use ultrasound-guided radial access: This has been shown to reduce the time to TRA, improve the first-pass attempt, and reduce the total number of attempts.7 Minimizing repeat radial artery needle punctures helps decrease arterial wall trauma. Further study is needed to determine whether ultrasound-guided access reduces the incidence of RAO.
- Use the patent hemostasis technique: Preservation of antegrade arterial flow while maintaining hemostasis lowers the incidence of early occlusion (12 percent compressive vs. 5 percent patent; p<0.05) and late occlusion (7 percent compressive vs. 1.8 percent patent; p<0.05) and, as such, is superior to traditional compression of the radial artery.8, 9
Figure 1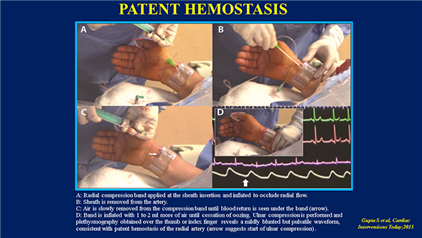
- Consider the use of prophylactic ipsilateral ulnar artery compression: The application of ulnar compression during the patent hemostasis technique has been shown to reduce RAO rates, compared with standard patent hemostasis (0.9 percent vs. 3 percent; p=0.0001).10 Essentially, a TR Band is positioned over the radial sheath entry site. Next, a compression band is placed over a piece of gauze applied over the ipsilateral ulnar artery in Guyon’s canal, and once plethysmography confirms ulnar artery compression, the radial sheath is removed and the TR Band is inflated using the patent hemostasis protocol.
- Use strategies to shorten the duration of radial artery compression: Typically, the radial band can be loosened 30 minutes after diagnostic procedures and 90–120 minutes after interventional procedures. The use of a topical hemostatic pad in conjunction with a radial band such as the TR Band lowered the duration of radial artery compression in a nonrandomized case series.11
Figure 2
RAO Management
Once RAO is suspected based on the absence of a previously palpable radial pulse, then confirmation should be obtained with either plethysmography or radial artery duplex ultrasonography. After confirmation of the diagnosis of RAO, treatment strategies are largely based on the presence or absence of symptoms, acuity of the RAO diagnosis, and need to maintain patency. Management strategies include conservative medical management with anticoagulation. Invasive management strategies to restore flow have been reported, but insufficient data exists to support their routine use. In addition, the following approaches, while not tested in large-scale studies, can be considered:
- Ipsilateral ulnar artery compression for one hour, as shown in Figure 4.TIF, may be beneficial particularly in patients who received 5,000 units of unfractionated heparin (UFH) during radial cardiac catheterization.12
- Anticoagulation with low-molecular-weight heparin (LMWH) for four weeks in patients with symptomatic RAO resulted in the recanalization of the radial artery in 87 percent of cases in one published study.13
Conclusion
The radial operator needs to be aware of the risk factors and prevention strategies for RAO. It is the responsibility of the physician to ensure all measures to prevent RAO are undertaken in the lab and the holding area. The most important measures include procedural anticoagulation, reduction of radial compression time, and use of the patent hemostasis technique. Novel strategies such as ulnar compression and the use of topical hemostatic agents, in addition to compression, have been reported and are being studied further.
References:
- 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016 Jan 14; 37(3): 267–315.
- Rashid M, Kwok CS, Pancholy SB, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016 Jan 25; 5(1).
- Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009 Oct 15; 104(8): 1083–5.
- Plante S, Cantor WJ, Goldman L, Miner S, Quesnelle A, Ganapathy A, et al. Comparison of bivalirudin versus heparin on radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv.
- Kwok CS, Rashid M Et al. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med. 2015 Dec; 16(8): 484–90.
- Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 1999 Feb; 46(2): 173–8.
- Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015 Feb; 8(2): 283–291.
- Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008 Sep 1; 72(3): 335–40.
- Gupta S, Nathan S. Radial Artery Use and Reuse. Preserving radial patency after transradial catheterization procedures. Cardiac Interventions Today. 2015 Jun.
- Pancholy SB, Bernat I, Bertrand OF, et al. Prevention of radial artery occlusion after transradial catheterization: the PROPHET-II randomized trial. JACC Cardiovasc Interv. 2016 Oct 10; 9(19): 1992–1999.
- MA Khuddus. Improving Patient Care and Post Procedure Efficiency Following Transradial Access. Cath Lab Digest. 2017
- Bernat I, Bertrand OF, Rokyta R, et al. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol. 2011 Jun 1; 107(11): 1698–701.
- Zankl AR, Andrassy M, Volz C, et al. Radial artery thrombosis following transradial coronary angiography: incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol. 2010 Dec; 99(12): 841–7.
Related QI Tips
Other evidence-based methods and tools you can use to improve quality of care and outcomes for patients.
