By: Kwan S. Lee, MD, FSCAI; Sandeep Nathan, MD, FSCAI; Huu Tam Truong, MD, FSCAI; Jayant Bagai, MD, FSCAI
Introduction
Transradial cardiac catheterization is usually performed via right radial access (RRA) due to the standard configuration of catheterization laboratories. Left radial access (LRA) is used less often, as it is not as ergonomic for the operator, especially for prolonged or complex percutaneous coronary intervention (PCI) and in obese patients. Given the benefits of the radial approach for PCI compared with the femoral approach, interventionalists must be familiar with unique advantages of LRA over RRA as well as with techniques that improve operator and patient comfort. Studies have shown significantly lower rates of subclavian tortuosity and slightly shorter fluoroscopy times with LRA compared to RRA.(1) Therefore, LRA should be considered in patients where tortuous vascular anatomy is expected (such as age > 75 years, height < 5’5’’).(2) LRA should also be considered as the preferred approach in patients with a patent left internal mammary graft. Operator radiation exposure to the head and thyroid is also lower with LRA compared with RRA.(3) Recently updated best practices recommend the choice between LRA versus RRA also be based on operator preference.(4)
In this Tip of the Month, we focus on setup, technical aspects of both proximal LRA (pLRA) and distal LRA (dLRA), and methods of hemostasis. In this article, which is not an endorsement of one approach over the other, pLRA refers to “conventional” LRA over the flexor aspect of the left wrist, while dLRA refers to the access in the anatomical “snuffbox.”
pLRA
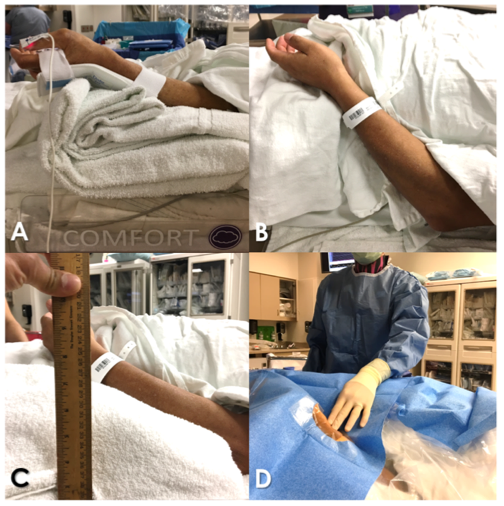
Figure 1: pLRA setup
- Setup for conventional LRA or pLRA is achieved with (Figure 1, A) or without (Figure 1, B) a flexible wrist support. The left wrist is propped on soft, supportive materials (Figure 1, C) with slight elbow flexion and partial hand pronation to ensure patient comfort and easy access from the right (Figure 1, D). The use of orthopedic finger traps or a hand strap, secured to the right side of the table, to leverage the arm toward the right and to avoid gradual slippage back has been described. Various devices have been used, such as the STARBoard radial arm board (Adept Medical Ltd. New Zealand).
- Most operators obtain pLRA from the left side of the table with the wrist supinated using sheaths in a standard 10 cm length. The hand is repositioned as noted above, and the operators perform the procedure from the right side. A minority of operators work from the left side, but this requires significant room and table reconfiguration.
- An alternative to the partial pronation technique is the use of a longer sheath (³ 16 cm) and a partial sheath insertion secured with clear adhesive. This increases exposure of the sheath access port and side arm for easier manipulation, allowing for more pronation and adduction to the right, reducing the reach required by the operator. One technique, described as “pseudoanatomical snuffbox access,” involves securing the sheath at the level of the snuffbox (Figure 2). Care must be taken to avoid kinking of the sheath. If this occurs, it can usually be corrected with a 0.035’’ wire and the insertion of a catheter.
- Hemostasis is obtained with standard techniques and devices.
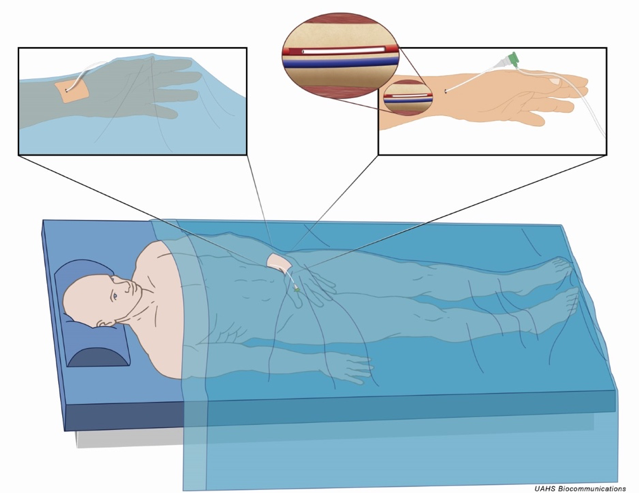
Figure 2: “Pseudoanatomical snuffbox access” using a partially inserted longer sheath
Distal Left Radial/Anatomical Snuffbox Access
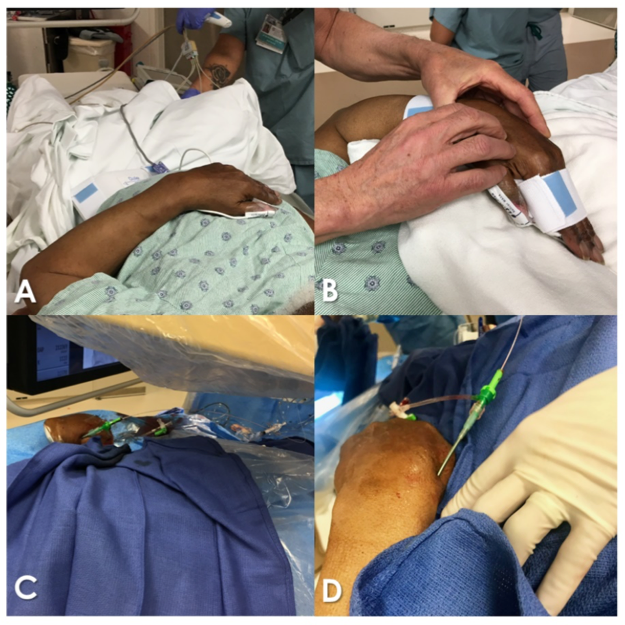
Figure 3: dLRA
- dLRA offers advantages of enhanced ergonomics and comfort for operator and patient. The hand may be prepped with full pronation, rested on the patient’s mid-to-low abdomen (Figure 3, A-D).
- A roll of 4” x 4” gauze or small towel can be held to keep the dorsal area opened, separating the thumb and first finger. The hand is prepped in this position and anesthetized, and access is gained—preferably with ultrasound. Through-and-through puncture is not recommended since needle contact with the periosteum is painful.
- Distal left radial hemostasis differs from proximal hemostasis. Common, rigid hemostasis bands depend on immobility of the wrist. The dorsal hand is more mobile, and these devices may be loosened by movement. One solution is to apply rolled gauze to the access site, secured by a tight elastic bandage for two hours. If a device is used, the dedicated distal radial PreludeSYNC DISTAL TM band (Merit Medical Systems, USA) has been suggested (Figure 4). Shorter hemostasis times may be observed.
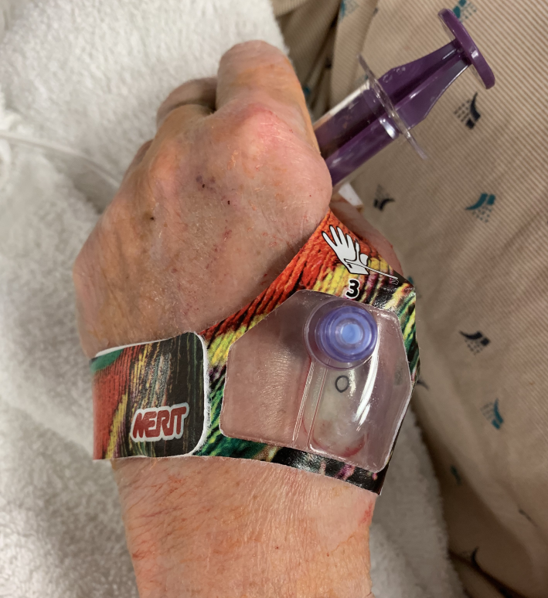
Figure 4: PreludeSYNC DISTAL band dedicated distal radial hemostatic device (Merit Medical Systems, USA)
- Possible benefits of dLRA over pLRA, beyond increased comfort, are reduction in the risk of forearm radial artery occlusion. This is because occlusion at the point of dLRA does not affect antegrade runoff through the superficial palmar arch, and this prevents proximal thrombus propagation. The risk of forearm compartment syndrome in the event of perforation is also lower. Other benefits include its ability to be used in patients with frozen shoulder who are unable to adduct their arm, as required for pLRA. Limitations include a smaller size of the vessel at this site, which makes access more difficult. The use of ultrasound is recommended in patients with a weak pulse.(5-7)
Summary
LRA access, at the proximal or distal level, has several advantages over RRA. Interventionalists must be familiar with methods to improve operator and patient comfort with LRA. Knowledge of the techniques mentioned in this Tip of the Month will allow operators and cath lab staff to overcome barriers to LRA and prevent crossover to femoral access in patients where RRA is not possible or unsuccessful.
References
- Norgaz T, Gorgulu S, Dagdelen S. A randomized study comparing the effectiveness of right and left radial approach for coronary angiography. Catheter Cardiovasc Interv. 2012 Aug 1;80(2):260-4.
- Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR, Crisco V, Woody W, Zoghbi G, Duffy PL, Sanghvi K, Krucoff MW, Pyne CT, Skelding KA, Patel T, Pancholy SB; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention’s transradial working group. Catheter Cardiovasc Interv. 2014 Feb;83(2):228-36.
- Kado H, Patel AM, Suryadevara S, Zenni MM, Box LC, Angiolillo DJ, Bass TA, Guzman LA. Operator radiation exposure and physical discomfort during a right versus left radial approach for coronary interventions: a randomized evaluation. JACC Cardiovasc Interv. 2014 Jul;7(7):810-16.
- Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, Drachman DE, Valle JA, Rhodes D, Gilchrist IC; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. An Update on Radial Artery Access and Best Practices for Transradial Coronary Angiography and Intervention in Acute Coronary Syndrome: A Scientific Statement From the American Heart Association. Circ Cardiovasc Interv. 2018 Sep;11(9):e000035.
- Kiemeneij F. Left distal transradial access in the anatomic snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). Eurointervention 2017 Sep 20;13(7):851-57.
- Davies RE, Gilchrist IC. Back hand approach to radial access: The snuff box approach. Cardiovasc Revasc Med. 2018 April-May;19(3, Part B):324-26.
- Corcos T. Distal radial access for coronary angiography and percutaneous coronary intervention: A state-of-the-art review. Catheter Cardiovasc Interv 2019 Mar 1;93(4):639-44.
Tips
Immediate Implementation Steps:
- Review the updated FDA letter to health care providers. Here is the link.10
- Have discussions with your patients prior to the procedure about PCDs. Patients should be informed about the potential long-term risks in the context of improving intermediate-term quality of life. Patient preferences and alternative therapies, including non-PCDs, should be discussed. Document this conversation in the medical record and in the procedural informed consent document. The basis for your conversation can be derived from the updated labeling in the Instructions for Use11 on currently available PCDs:
“A signal for increased risk of late mortality has been identified following the use of paclitaxel-coated balloons and paclitaxel-eluting stents for femoropopliteal arterial disease beginning approximately 2-3 years post-treatment compared with the use of non-drug coated devices. There is uncertainty regarding the magnitude and mechanism for the increased late mortality risk, including the impact of repeat paclitaxel device exposure. Physicians should discuss this late mortality signal and the benefits and risks of available treatment options with their patients.”
- Limit your use of PCDs to select patients who are at high risk for restenosis and repeat femoropopliteal interventions and when the patient feels the benefits would outweigh the risk of late mortality. Having a discussion prior to the procedure to set this context will ensure a patient-centered approach to the ideal goals of therapy.
- Provide close follow-up along with optimal peripheral artery disease (PAD) medical therapy, including counseling on smoking cessation and exercise.
- Consider reporting any adverse events noted periprocedurally or during follow-up to the FDA via MedWatch, the FDA Safety Information and Adverse Event Reporting Program.12
A. Pre-PCI Considerations
- Assess the need for PCI. Does the patient really need a stent? The 2017 appropriateness use criteria (AUC) for PCI can help to guide decision-making.
- Assess the risk of stroke. Long-term OAC is recommended for CHA2DS2-VASc > 2 in men and > 3 in women.10
- Assess the risk of bleeding. A HAS-BLED score of >3 is associated with a high bleeding risk but is not a reason to withhold anticoagulation. Instead, modification of risk factors and more cautious monitoring is recommended in such patients.
B. Considerations During PCI
- Use radial access preferentially over femoral access for PCI in patients who require post-PCI anticoagulation.
- Note that studies have shown the safety of four weeks of DAPT in patients at a high risk of bleeding after implantation of newer generation drug-eluting stents (DES), with superior efficacy compared to bare-metal stents (BMS).11, 12 Therefore, the use of BMS to lower DAPT duration can no longer be justified.
- Ensure adequate clopidogrel and aspirin loading pre-PCI in all patients. Continuation of aspirin until hospital discharge is reasonable and should be considered even in patients in whom DT is planned on discharge.
C. Considerations After PCI
- DT vs. TT
- The decision between DT and TT depends on the balance between ischemic risk (acute coronary syndrome (ACS), complex PCI, stent length > 60 mm, prior stent thrombosis (ST) or high DAPT score and bleeding risk. A summary, based on the 2017 European Society of Cardiology (ESC) guidelines and expert opinion from North America, is presented in Table 1.
- WOEST was the first trial to show that omission of aspirin from TT resulted in a highly significant 25 percent absolute risk reduction (number need to treat or NNT =4) without an increase in ischemic events (death, myocardial infarction [MI], stroke, revascularization, or ST). Limitations of this study included an open-label design and lack of power to assess for the difference in ST. In ISAR-TRIPLE, six weeks compared with six months TT was not associated with an increase in ischemic events but lowered the incidence of BARC 2-5 bleeding between six weeks and six months. In both these trials, the rate of ACS and complex PCI was low.1, 3
- In the AUGUSTUS trial, TT was associated with a significant increase in the risk of bleeding at six months (HR 1.89, number need to harm or NNH=14) compared to DT. Omission of aspirin lowered bleeding by 47 percent.6
- Antiplatelet agent considerations
- Most patients enrolled in recent studies were taking clopidogrel. Ticagrelor was used as part of DT in 12 percent of patients in RE-DUAL PCI. However, prasugrel and ticagrelor should not be used as a component of TT, as they significantly increase the risk of bleeding (Class III-harm, per 2017 ESC guidelines).
- Aspirin dose should typically not exceed 81 mg.
- Consider discontinuation of the antiplatelet agent from DT after one year in patients with low ischemic risk and after six months in patients with a high bleeding risk.
- Anticoagulant considerations
- A summary of recent RCTs comparing DT and TT, including regimens comparing direct oral anticoagulants (DOAC) and warfarin, is presented in Table 2.
- Both the ESC guidelines and North American expert consensus document recommend using a DOAC instead of warfarin if there is no contraindication. It is reasonable to continue warfarin if the patient was tolerating it or if creatinine clearance is < 30 ml/min. The international normalized ratio (INR) target should be between 2-2.5.
- There is no role of withholding OAC in patients with AF post-PCI and treating them with just DAPT.13
- DOACs are not approved for “valvular AF,” which is defined as AF in the presence of a mechanical heart valve or moderate-to-severe mitral stenosis.
- Tips to lower bleeding
- The following can lower bleeding: the routine use of proton pump inhibitors for gastric protection, avoidance of supra-therapeutic INR, blood pressure control, adjustment of DOAC dose based on creatinine clearance, patient education on signs and symptoms of bleeding, avoiding nonsteroidal anti-inflammatory drugs (NSAIDs) and alcohol, and closer monitoring of patients on TT and those with a HAS-BLED score >3.
Related QI Tips
Other evidence-based methods and tools you can use to improve quality of care and outcomes for patients.
