Why is the study important?
Despite the robust level of data that has been accumulated over the last decade in favor of transcatheter aortic valve replacement (TAVR), several questions remain regarding the durability of transcatheter heart valves (THV). Initial 4-year results of the Evolut Low Risk Trial at TCT 2023 were encouraging but 17.7% of bioprosthesis implanted in the surgical aortic valve replacement (SAVR) arm were Trifecta bovine pericardium valves, which have been withdrawn from the market due to durability concerns. This study examined the 4-year Evolut Low Risk Trial analysis excluding SAVR who received the Trifecta valve.
Should I change my practice because of these findings?
The study continues to build on the growing body of evidence supporting the medium-term durability of TAVR however longer-term data will be needed.
What question was the study supposed to answer?
What are the 4-year results of the Evolut Low Risk Trial excluding patients who received a Trifecta bovine pericardium valve?
What did the study show?
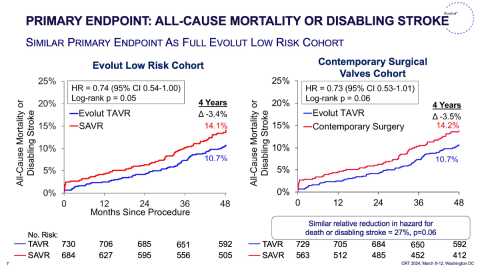
The Evolut Low Risk trial was a multicenter, randomized controlled trial assessing the safety and efficacy of TAVR using either the CoreValve Evolut R or CoreValve Evolut PRO THV compared to SAVR. The initial study design included 17.7% Trifecta SAVR valves, thus this analysis of the Evolut Low Risk trial excluded those patients, comparing 729 patients who underwent TAVR to 563 patients who underwent SAVR, demonstrating that a 4 years all-cause mortality or disabling stroke were similar (10.7% vs. 14.2%; HR, 0.73; 95% CI,0.53,1.01; p=0.06). Findings were similar compared to the initial 4-year Evolut Low Risk analysis. Rates of permanent pacemakers (23.8% vs. 9.7%; p<0.001) remained high in the TAVR arm as did rates of atrial fibrillation (40.8% vs. 14.0%; p<0.001) in the SAVR arm. The incidence of having a mean gradient greater than 20mmHg remained higher in patients who underwent SAVR at 4 years (8.9% vs. 4.0%; p=0.002).
How good was the approach/methodology?
This was a retrospective post-hoc sub-group analysis of the Evolut Low Risk trial, with inherent limitations not necessarily powered adequately to detect significant differences.
Other Specialist Resources for Structural Heart Disease
Including recently published studies, coverage of late-breaking science, updates from clinical trials and registries, and complex case presentations.