Why is the study important?
Early outcomes in the prospective, multicenter, international, single-arm Navitor investigational device exemption (IDE) study are sustained out to 1 year in patients at high- or extreme-risk for surgical aortic valve replacement (SAVR) using the second-generation Navitor transcatheter heart valve (THV).
Should I change my practice because of these findings?
The Navitor THV continues to perform well among patients at high- or extreme-surgical risk out to 1 year, however longer-term and comparative data will be needed to elucidate any clinical differences that may exist between the Navitor THV and other commercially available THVs.
What question was the study supposed to answer?
This study sought to examine the 1-year performance of the Navitor THV in patients deemed to be at high- or extreme-risk for SAVR.
What did the study show?
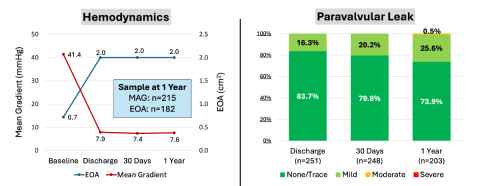
The 1-year analysis of patients enrolled in the prospective, multicenter, international, single-arm Navitor IDE study demonstrated 6.6% all-cause mortality, 2.3% disabling stroke, 6.6% life-threatening bleeding, 21.7% permanent pacemaker rate and 0.4% reintervention rate. Hemodynamics remained similar (30 days: 7.4mmHg and 1 Year: 7.6mmHg) as did aortic valve areas (30 days: 2.0cm2 and 1 Year: 2.0cm2) and rates of moderate or severe paravalvular leak (30 days: 0.0% and 1 Year: 0.5%). Additional 5-year data was presented examining the performance of the first-generation PORTICO, demonstrating a mean gradient of 6.7mmHg in the adjudicated registry (n=941) and 7.2mHg in the PORTICO IDE trial.
How good was the approach/methodology?
The Navitor IDE study was well-designed to demonstrate the safety and efficacy of the Navitor THV in patients deemed to be at high- or extreme-risk for SAVR in the short term.
Other Specialist Resources for Structural Heart Disease
Including recently published studies, coverage of late-breaking science, updates from clinical trials and registries, and complex case presentations.